1. Introduction
The pharmaceutical industry has constantly been evolving in terms of development strategies and base the delivery of their therapeutic agents on two pivotal factors: i) developing medicines to be as selective as possible to the site of action in the body ii) maintaining a constant reproducible right concentration of drug at the right place in the body that reduces dosage frequency (Kingsley et al., 2006). The significance of engineering therapeutic agents to be target oriented is to minimize the emergence of any possibilities for adverse and life-threatening effects as is the case for cytotoxic compounds (Kingsley et al., 2006). Paul Ehrlich articulated the theory or concept of drug targeting back to 1891 (Kingsley et al., 2006).
Nanotechnology is a fairly newly established field in the era of science that engages multi-scientific disciplines including chemistry, biology, engineering and physical sciences (Kumar 2000; Martín del Valle et al., 2009). There is not an exclusive universal definition for nanotechnology, however, the National Nanotechnology Initiative (NNI) defines nanotechnology as the study of matters whose one dimension at least is between 1 to 100 nm as the materials exhibit unique properties at nanoscale (Balogh, 2010).
A well-known lecture “there is plenty of room at the bottom” delivered by Nobel winner Richard P. Feynman in 1959 (Khan et al., 2019) was a pioneer speech of the manipulation of nano-size matters concept. Since then, there have been enormous advances in this field with the first nanoparticles developed around 1970 with its primary use as carriers in the delivery of vaccines and cytotoxic drugs (Kumar 2000). Nano-structures have gained recent popularity as promising routes in overcoming the limitations of conventional drug treatments such as a drug’s poor aqueous solubility, unwanted side effects causing cytotoxicity and the short longevity of drug presence in the body.
It is estimated that around 70% of newly discovered drugs are water insoluble and about 40% of the currently used oral immediate-release drugs are also water-insoluble (Amoabediny et al., 2018). The conventional drug delivery treatments have always been faced with the challenges of poor drug aqueous solubility that results in reduced bioavailability and biodistribution effects (Cartaxo, 2015). Many of the potential drug candidates showing exceptional in vitro therapeutic effects are rejected due to their poor physiochemical properties (Amoabediny et al., 2018). This implies that pharmaceutical industries are working towards the development of new bioactive molecules to benefit novel therapies, but also need to address the mechanisms by which they will be delivered to the body (Martín del Valle et al., 2009). This brings to light the significant role of research work carried out in the field of drug delivery systems (DDS).
Nanocarriers can be utilised to obtain the goal of securing optimal therapeutic effects whilst at the same time minimizing the unwanted side effects (Moghimi et al., 2005). Owing to the exceptional ratio of surface area of atoms/molecules to their mass/ volume, nanoparticles are capable of penetrating to the most inaccessible sites and tiny capillaries in the body (Jain & Thareja, 2019). This feature is rightly exploited in the case of cancerous cells due to the enhanced permeability and retention (EPR) effects, lack of effective lymphatic drainage, and also when the permeability mediators are growing more rapidly (Kingsley et al., 2006; Jain & Thareja, 2019). As EPR effects are solely existent in solid tumour cells and not in healthy ones, it guides nanoparticles to a more targeted approach (Kingsley et al., 2006).
This mini-review focuses on mainly four types of organic nanocarriers including micelles, compact polymerics, solid-lipid nanoparticles (NP) and liposomal vesicles. This comprises of definitions, classifications, methodologies and evaluation techniques. There is also a special focus on how each component has evolved over the past three decades. Some of the challenges associated with earlier NPs are also discussed.
2. An overview of multifunctional nanocarriers
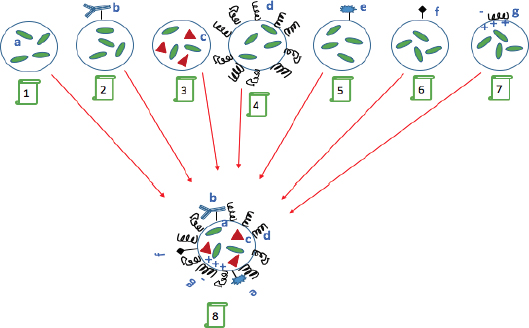
Since the development of mono-functioning nano-carriers the next generation was introduced as “multi-functional” nano-particulates. This is still a growing field that is being explored. The surface properties of nano-carriers control their function, hence by modifying the surface of carriers in a favourable fashion, one can make them possess simultaneous performances as the name “multi-function” suggests (Torchilin, 2007). The aim is to simultaneously obtain several major benefits in an orchestrated way: i) to prolong the longevity of carrier/drug presence in the blood circulation ii) targetability by attaching specific ligands such as peptides, transferrin and antibodies to the NPs iii) to create stimuli- sensitive nano-carriers (e.g. pH or temperature sensitive by use of appropriate lipids or polymers) iv) to utilise them as contrast/diagnostic agents for organ or tissue imaging (by loading on nano-carriers heavy metal atoms such as 111In, Tc, Gd and Mn (Torchilin, 2007; Torchilin, 2012). Figure 1 illustrates the aforementioned purposes and multi-functionality of nano-carriers.
1, Conventional active pharmaceutical ingredient (API) loaded nanocarrier. 2, modified nanocarrier surface with targeting agents. 3, addition of magnetic particles into the nanocarrier to induce carrier response to the surrounding magnetic field. 4, attachment of long circulating polymers such as poly (ethylene glycol) (PEG) to enhance drug blood circulation time. 5, attachment of contrast agents for imaging applications. 6, enhancing the nanocarrier cell penetration by attaching cell penetrating peptides onto the surface. 7, complexing negatively charged DNA onto the positively charged surface of nanocarrier. 8, The combination of all 1-7 functions converged on a so called multifunctional nanocarrier (adapted from Torchilin, 2007).
3. Nanostructures
Manipulating solids at the atomic level transforms the properties of bulk material (Gleiter, 2000). Changes in microstructures comprises of their size (0D, 1D, 2D, 3D), structure of atoms and chemical composition (Gleiter, 2000). For instance, bulk gold (Au) is resistant to tarnishing for thousands of years whilst nano-particles of Au exhibit high level of reactivity and are capable of producing heat from absorbing and conversion of light energy. They can as such, be exploited for pharmacological purposes (Dreaden et al., 2012).
3.1 What is precisely referred to as “nano-particles”?
Some of the reviewed literature accord the prefix “nano” (meaning dwarf) to any matter with the size of less than 100 nm in at least in one dimension (Khan et al., 2019; Laurent et al., 2008; Roco, 1999), whilst others stretch the size range to 10–1000 nm (Kingsley et al., 2006). The British Standards Institution (2005) characterized nanoparticles with the size of less than 100 nm at the point which their properties differ from the bulk scale. This is in contrast to the nanoparticle sizes (100-500 nm) used in pharmaceutics (Buse & El-Aneed, 2010). Whilst there are long-standing debates over the size threshold, an accurate way to distinguish nanoparticles is by looking at whether they are ruled by quantum effects or Newotonian physics (Buse & El-Aneed, 2010). A particle whose behaviour is based on quantum mechanics is classified as nanoparticle (Buse & El-Aneed, 2010). It is beyond the scope of this review to delve into defining particles in the field of quantum mechanics (e.g. wave-particle duality concept) and the correlation of particle size (or mass) at nano-scale with its de Broglie wavelength or wavelength of particle motion. There are however, excellent textbooks such as “101 Quantum Questions” (Ford et al., 2011) that readers are directed for better understanding of those concepts.
3.2 Classification of pharmaceutical nano-particulate systems
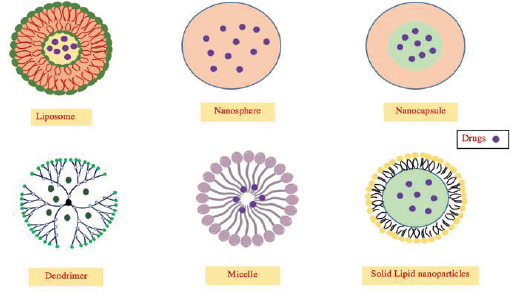
Pharmaceutical nano-particles can be classified based on distinct categories depending on any of their chemical composition, morphology or their applications. Figure 2 is a comparison of two separate methods of their classification.
Classification of nanoparticles (NPs) (adapted from (a) Cartaxo, 2015 and (b) Jain and Thareja, 2019).
3.2.1 Micelles
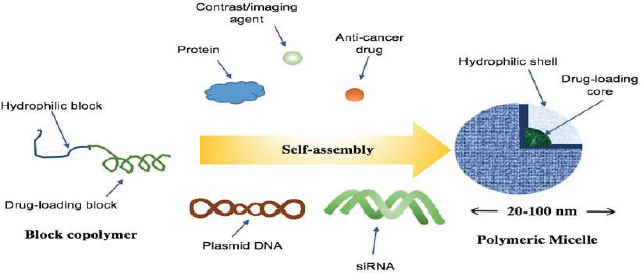
Micelles are nano-sized vectors that are capable of self-assembly when exposed to aqueous solutions (Orive et al., 2010). Both lipids and polymers could be composed of amphiphilic molecules acting like a micelle, organized with a hydrophobic block (the core space) and a hydrophilic block (the corona shell) (Figure 3) (Miyata et al., 2011). As the result of hydrophobic interactions as the main driving force, amphiphilic block copolymers in aqueous milieu self-arrange so that the drug-loading core is concealed inside and the hydrophilic shell is thus exposed outside (Figure 4) (Cartaxo, 2015; Miyata et al., 2011). The entrapment of drug into the core can be either by hydrophobic interactions or more specifically by covalent bonding to the core domain (Miyata et al., 2011). Polymeric micelles contain a polymeric core composition whereas lipid-core micelles are formed from two long chain fatty acyl groups as the hydrophobic block that render the core extremely hydrophobic (Torchilin, 2007). Various hydrophilic block polymers can be used from which poly (ethylene glycol) (PEG) is the most popular candidate due to it mainly being a good steric protector against biological degradation and providing further surface stabilisation along with other benefits (Torchilin, 2007; Dalia, 2015). An important parameter in micelles synthesis is their critical micelle concentration (CMC) and critical micelle temperature (CMT). CMC is the concentration at which micelles start aggregating from individual amphiphiles and CMT is the temperature above which micelles remain aggregated and under which as monomers (Torchilin, 2007).
Self-assembly of micelles in aqueous solution (adapted from Miyata et al., 2011).
Structures of a number of nano-carriers in DDS (adapted from Orive et al., 2010).
Various types of drugs, mainly sparingly soluble anti-cancer medicines, proteins and genes can be loaded into the hydrophobic core of the amphiphilic block copolymer (Torchilin, 2007; Kataoka et al., 2001). Polymeric micelles have shown superior properties and characteristics in comparison to the conventional surfactant micelles, owing to their lower CMC value (that brings them more stability in solutions) and a prolonged dissociation rate that extends the drug’s blood circulation time (Kataoka et al., 2001).
Forster Resonance Ene.g., Transfer Technique (FRET) is an effective technique used to study the stability of polymeric micelles (Cho et al., 2013). This technique was exploited in monitoring the release of hydrophobic molecules entrapped into the core from polymeric micelles into cell membranes (Chen et al., 2008). FRET measures the ene.g., transfer (ET) that occurs between a donor chromophore and an acceptor chromophore, which is inversely proportional to their distance to the power of six (ET = 1/R6). Chen et al. (2008) loaded two hydrophobic dyes (di-alkyl indocarbocyanine (DiIC18) as an acceptor and DiOC18 as a donor) into polymeric micelles and observed the changes in FRET signals as the core-loaded micelles were incubated with KB cells for 2 h. When the FRET signal was observed, this implied that the two hydrophobic dyes were in proximity held within micelle core, however, once the dyes were released from micelle or when micelle was decomposed there would be an absence of FRET signal (Chen et al., 2008).
Polymeric micelles demonstrate an enhanced drug biodistribution preceded by an increase in the bioavailability of a drug across physiological barriers (Parveen et al., 2012). Polymeric micelles have a high capacity for drug loading and can be utilised to make sustained release drugs (Parveen et al., 2012) for example, by controlling the degradation rate of the hydrophilic block that determines the liberation of drug from the core (Torchilin, 2007). There are a number of poorly soluble drugs that have been successfully formulated by polymeric micelles e.g anthracycline antibiotics, diazepam, adriamycin and polynucleotides (Parveen et al., 2012). In addition, one can make specific targeting micelles by attaching them on specific ligands such as antibodies, folate or transferrin (Torchilin, 2007).
3.2.2 Compact Polymerics
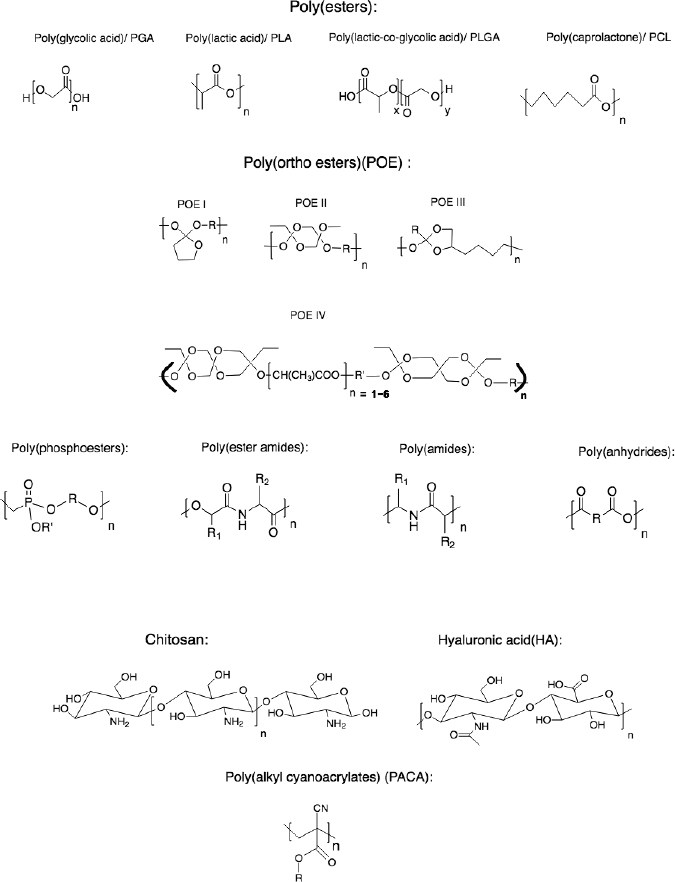
Polymeric nano-carriers (PNCs) are either synthetically or naturally sourced colloidal particles with widespread applications in drug delivery systems (DDS). Examples of naturally existent polymers are protein-based polymers including gelatine, collagen and albumin, or polysaccharides e.g., alginate, chitosan, agarose and hyaluronic acid (HA) (Kamaly et al., 2016). Synthetic polymers are more attractive in DDS due to being more reproducible and showing less batch-to-batch molecular weight (MW) distribution and variation as opposed to their natural counterparts (Kamaly et al., 2016). Figure 5 illustrates some of the polymers used as nano-carriers.
PNCs can be subdivided into two types based on their preparation methodologies: i) nanospheres ii) nanocapsules. Nanospheres are composed of solid matrix that is solid in its total mass (Vauthier & Bouchemal 2009). The name “sphere” does not necessarily denote a spherical shape though most are spherical. There are, however, some described non-spherical particles in the literature that fall into this category (Vauthier & Bouchemal, 2009; Vauthier & Couvreur, 2000; Raoa & E. Geckeler, 2011). A drug can be strongly adsorbed at the nanospheres’ surface, entrapped or dissolved into the solid matrix (Sahoo & Labhasetwar, 2003).
On the other hand, nanocapsules bearing a vesicular-like structure such as a reservoir containing liquid (oil or water) or semisolid materials in the core that is surrounded by a solid polymeric shell (Vauthier & Bouchemal, 2009; Raoa & Geckeler 2011). The drug is again either adsorbed at the surface or entrapped inside cavity of the core (Vauthier & Bouchemal 2009). Molecules with a high degree of sensitivity are best to be entrapped at the core to prevent them from biological degradation (Vauthier & Bouchemal, 2009). This requires drug association to the carrier during the preparation stage (Vauthier & Bouchemal, 2009). However, if the drug is highly sensitive to degradation during the preparation process, or does not associate to its carrier at that stage, it is loaded by adsorption to the nanocapsules (Vauthier & Bouchemal, 2009).
From the synthetic polymers utilised in DDS, the most commonly used are saturated poly (α -hydroxy esters) such as poly (glycolic acid) (PGA), poly (lactic acid) (PLA) and poly (lactic-co-glycolic acid) (PLGA) copolymers (Lombardo et al., 2019).
The aforementioned polymers in Figure 5 are particularly favoured due to their good profile of biocompatibility and low toxicity along with a control over their rate of degradation in vivo (Lombardo et al., 2019).
For instance, it is worth considering that how stereochemical centre in poly (lactic acid) (PLA) and poly (D, L-lactic-co-glycolic acid) (PLGA) affect polymer degradation rate following the rate of drug liberation.
The asymmetric α-carbon in PLA have laevorotatory (PLLA) and dextrorotatory (PDLA) enantiomers i.e., if all the stereocentres of PLA are L figured is termed PLLA (Kamaly et al., 2016). The PLLA enantiomer is crystalline and the PDLA enantiomer is amorphous and poly (glycolic acid) (PGA) is crystalline (Kamaly et al., 2016). Hence, properties such as crystallinity and amorphousness can be tuned by varying the ratio of lactide to glycolide as well as D and L isomers of PLA in PLGA synthesis (Kamaly et al., 2016). Zilberman (2005) studied the release profiles of the drug dexamethasone (DM) from different enantiomers of bioresorbable polymer films including PLA and PLGA. It was shown that the rate of drug release was directly dictated by the polymer degradation (or weight loss) rate. It was also concluded that, whilst there were other factors such as the shape of the created porous structure through degradation influencing drug release from the film, at the time of low percentage of weight loss of polymer (e.g. 5%), amorphous systems saw a higher rate of drug release compared to the crystalline systems (Zilberman, 2005). However, that conclusion is not valid at the higher percentage (e.g 10% and 20%) of polymer weight loss (Zilberman, 2005).
3.2.2.1 Preparation techniques of polymers in DDS
There are several ways to categorise PNCs preparation techniques with various terminologies. A number of reviewed papers describe methodologies as i) top-down approach (e.g. media milling, high-pressure homogenisation and lithography), ii) bottom-up approach (e.g. self-assembly and chemical synthesis of nanoparticles) (Khan et al., 2019; Buse & El-Aneed, 2010). Others provide a more detailed description of techniques as to whether starting with monomers or preformed polymers (macromolecules). In the former case, a polymerisation process is required (Pinto Reis et al., 2006). There are two techniques in polymerisation i) emulsion polymerization. This can be organic or aqueous ii) interfacial polymerisation (Pinto Reis et al., 2006). With preformed polymers, the commonly used techniques include: emulsification-solvent evaporation, emulsification-solvent diffusion, salting-out, dialysis, nanoprecipitation (solvent-displacement) and supercritical fluid technology (Pinto Reis et al., 2006; Crucho & Teresa Barros, 2017; Mora-Huertas et al., 2010).
3.2.2.2 Characterisation of PNCs
The particles size, morphology and polydispersity can be observed by scanning electron microscopy (SEM), transmission electron microscopy (TEM) and photon correlation spectroscopy (PCS) known as dynamic light scattering (DLS) (Crucho & Teresa Barros, 2017; Nagavarma Namuri et al., 2013). Another powerful technique is atomic force microscopy (AFM) with applications not only to measure PNCs size dispersity and aggregations but also as a non-destructive tool with the ability to measure force interactions at the molecular level on a single cell surface in real time (Dufrêne, 2002). The force measurement results from AFM can also provide insights to mechanical properties and microbial cell surface charges (Dufrêne, 2002).
The nanoparticles surface charge that is articulated as zeta-potential (ζ) in colloidal research, is commonly measured by a Zetasizer instrument (Nagavarma Namuri et al., 2013). Whilst zeta potential and size are two different concepts, both can sometimes be measured by the same instrument at a single run.
In the following, a brief description is given to aid the understanding behind the principles of zeta potential and its importance.
Zeta potential (ZP)
Surface charge of NPs is one of the important factors that has direct effects over toxicity and the extent of cellular uptake of nanoparticles causing their intended biological activity (Skoglund et al., 2017). This is so, as NPs surface charge determines their interaction with the surrounding biological media (Skoglund et al., 2017). For instance, it has been shown that cytotoxicity of metal-containing NPs varies depending on the value of ZPs (Skoglund et al., 2017).
The potential of the actual particle surface that is termed Nernst (ψ0) potential is not measurable (Vidal-Iglesias et al., 2012). However, what can be measured is the potential difference between the electric double layer (EDL) of NPs and the surrounding moving particles during electrophoresis at the shear plane (Bhattacharjee, 2016). EDL is the adsorbed layer formed around a charged particle when dispersed in an electric field and shear plane is the interface between EDL and moving particles in dispersion (Bhattacharjee, 2016).
Generally, electrophoretic light scattering is a common method in zeta potential instruments.
From this technique, particles velocity is obtained while applying an electric field and that value is then used to calculate zeta potential through several mathematical equations (Bhattacharjee, 2016). Detailed accounts of operational mechanisms of zeta potential instruments are beyond the scope of this review, although there are excellent reference literatures for a better understanding of their operations (Kvasnov et al., 2019).
Early research reported that nanoparticle concentration at low levels could directly affect the reproducibility and reliability of data sets obtained from zeta-potential and DLS (for particle size measurements) which can be particularly concerning for those studying NP’s toxicity (Tantra et al., 2010). As a result of NPs’ small size and massive surface area to volume ratio, they have the ability to reach and access tissues at the cellular level inducing potential reactivity (Bakand & Hayes, 2016). This therefore raises the probability of the emergence of any potential toxicity (Bakand & Hayes, 2016).
Toxicological experiments are often carried out in small NPs’ concentrations on cell models in order to determine the extents of cytotoxicity effects (Tantra et al., 2010; Garcia-Fuentes & Alonso, 2012). For example, chitosan’s half maximal inhibitory concentration (IC50) is between 0.2 to 2 mg/mL and so much depends on its molecular weight (Garcia-Fuentes & Alonso, 2012). One should therefore avoid basing experimental results’ interpretation on inaccurate data sets obtained from instruments.
A study concluded that the concentration effects of NPs on zeta-potential and DLS data reproducibility was not the case within a concentration range predicated based upon the nature of the sample in question (Tantra et al., 2010). In other words, one should experimentally work out a sample’s concentration range to provide consistent data and no shifts in zeta-potential and particle size values over replicates (Tantra et al., 2010).
3.2.3 Solid lipid nanocarriers
Solid lipid nanocarriers (SLNs) are the first series of formulated lipid-based nanocarriers (Ghasemiyeh & Mohammadi-Samani, 2018). They are produced with lipids, water, stabilising surfactants and co-surfactants (Yoon et al., 2013). If the lipid used is in solid state, it is classified as SLN, and if a mixture of solid and liquid lipids are used, it is termed nanostructured lipid carriers (NLCs) (Yoon et al., 2013). In either case, lipids remain solid at both room and body temperatures (Yoon et al., 2013). There are many superior properties about solid lipid-based nanoparticles (SLBNs: SLN and NLC) over liposomes and other emulsion systems from which that which makes them so attractive is their derivation from physiologically friendly lipids and thus allowing an organic solvent-free preparation process (that both results in low potential toxicity) along with simplistic large scale-up production procedures (Ghasemiyeh & Mohammadi-Samani, 2018; Yoon et al., 2013; Mehnert & Mader, 2001). SLNs are prepared by techniques such as high shear homogenization, ultrasound, high pressure homogenization (HPH) (cold and hot), along with other solvent emulsions and microemulsions techniques (Mehnert & Mader, 2001). HPH is an economical technique that facilitates scaling up (Mehnert & Mader, 2001). It demonstrated no issues in homogenizing lipid concentrations as high as 40% (Lippacher et al., 2000). SLNs produced by this technique contained less microparticles in dispersion as is a problematic case with high shear homogenization and does not represent the concerns of metal contamination by ultrasound (Mehnert & Mader, 2001). Although, it needs to be considered that particle sizes are highly affected by the type of emulsifiers homogenized in HPH technique (Mehnert & Mader, 2001).
One of the main concerns in the earlier use of lipid particles as nanocarriers was their suitability as prolonged drug release devices in DDS (Zur Mühlen et al., 1998). Zur Mühlen et al. (1998) showed that the SLNs release profiles were more dependent on the nature of the incorporated drug. In 2014, a novel method of SLNs preparation as “microwave-based microemulsion technique” was proposed with the results indicating enhanced SLNs characteristics (Shah et al., 2014).
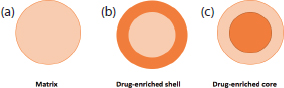
There are three models used to incorporate APIs into SLNs (Figure 6). In cold homogenisation, the product is mainly a homogeneous matrix, or when a drug with high lipophilic characteristics is mixed with SLNs in hot homogenisation (Muller et al., 2002). An API-enriched shell and API-enriched core are both resulted during phase separation (lipid/drug) at cooling stage in hot homogenisation (Muller et al., 2002). It can be summarised that if the lipid starts solidifying first, the drug is most concentrated in the shell, whereas if the drug crystallises first the drug-enriched core will be observed (Muller et al., 2002).
(a) homogeneous matrix, (b) API-free core and drug-enriched shell, (c) 4 drug-enriched core (adapted from Muller et al., 2002).
3.2.4 Liposomes
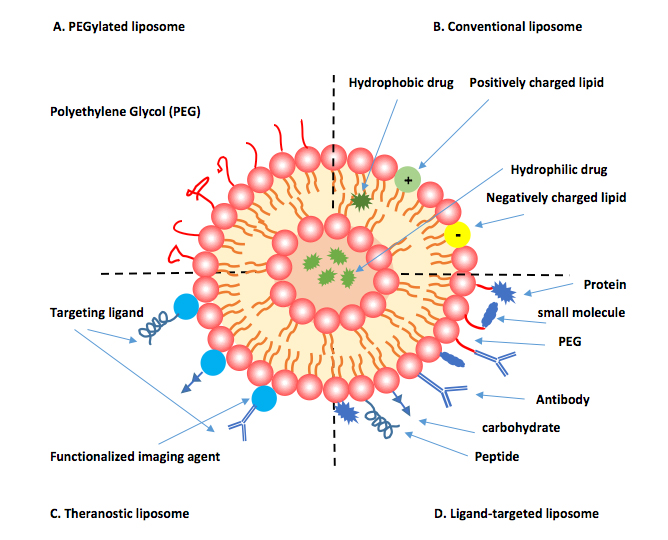
Liposomes are spherical vesicles composed of amphiphilic bilayered phospholipids that re-arrange themselves in aqueous milieu (Houshmand et al., 2020). They can present (small or large) unilamellar or multilamelar phospholipids bilayers surrounding a hydrophilic core and can load either hydrophobic or hydrophilic drugs (Houshmand et al., 2020). The choice of phospholipid type determines the liposome’s surface charge (neutral, cationic, anionic) (Houshmand et al., 2020). Since 1987, cationic liposomes have been employed for gene delivery by complexing negatively charged DNA into the vesicle due to the electrostatic interactions that indicate a higher capacity for gene loading over viral vectors (Lasic, 1997). Liposomal drug delivery has also found significant applications in cancer chemotherapy, mainly due to the alteration of a drug’s pharmacokinetics when associated to the liposomal system (Allen, 1997). A commonly used strategy that substantially enhances the blood half-life of a liposome-loaded drug up to 45 h (regardless of dose) is PEG surface coating (Allen, 1997; Suggy S et al., 2002) (Figure 7). PEG-coated liposome-loaded anthracyclines including doxorubicin and daunorubicin are well-studied examples of Food and Drug Administration (FDA) approved and commercialised drugs (Allen, 1997; Gabizon et al., 1998).
A pictorial representation of types of liposomal surface modification (adapted from Houshmand et al., 2020).
The preparation methods of drug loading into liposomal systems fall into two types of techniques: i) passive loading techniques ii) active loading techniques, that each divide into further types of methods and is extensively covered in (Akbarzadeh et al., 2013).
4. Clinical applications of nanoparticulate systems
Over the past years, there have been several successfully marketed nanoparticulate formulations, in particular, liposomal-associated medicines that exhibit superior therapeutic profiles compared to that of their conventional formulations. Nevertheless, the controversial reports on the toxicity aspects of these formulations in literature suggest that further investigations in this field are warranted.
The intravenously administered antibiotic amphotericin B (AB) is often employed as the second or third line of treatment for the vector-born leishmaniasis disease (Wijnant et al., 2018). AB is the choice of treatment in the case of severe therapeutic failure to the first line of treatments (e.g., pentavalent antimonials) (Wijnant et al., 2018). There are 20 species of protozoan leishmania genus attributed to the disease with various clinical implications from cutaneous to visceral leishmaniasis (Burza, Croft, & Boelaert, 2018). Rodríguez Galvis et al. (2020) controlled-trial study reported that the complexation of AB into the lipophilic liposomes (L-AB) reduced the adverse toxic effects on kidneys and liver that could be problematic in the conventional AB deoxycholate (ABD) (Rodríguez Galvis, Pérez Franco, Casas Vargas, & Ordoñez Rubiano, 2020). The liposomal complex caused less renal drug uptake and greater accumulation of AB at the target site that manifested itself in less toxicity and an increase in drug potency for the treatment of visceral leishmaniasis (Rodríguez Galvis, Pérez Franco, Casas Vargas, & Ordoñez Rubiano, 2020). A randomised double-blind trial study in 2000 also reported less infusion-related reactions on day 1 after L-AB administration compared to ABD formulations (Wingard et al., 2000).
However, Lindquist et al. (2020) recently reported a rare case of development of dystonic reactions in a patient–diagnosed with Candida glabrata–after 10 min of L-AB infusion; this was not observed when the treatment switched to ABD administration (Lindquist, Poveromo, Vann, & Wrenn, 2020). The authors believed that dystonia had not been previously listed in the L-AB adverse effects from the manufacturer’s labelling. Moreover, Kullab et al. (2020) reported the first case of the emergence of non-occlusive ST-segment elevated myocardial infarction (STEMI) after 24 h of L-AB infusion in a patient with cryptococcal meningitis. The report concluded that the application of L-AB for patients with cardiopathy should be cautiously considered despite less infusion-related reactions generally associated with L-AB formulations (Kullab, Patel, & Lewis, 2020).
In chemotherapy, the anthracycline drug classes such as doxorubicin and daunorubicin have been the first line of effective treatment in broad use for many cancers since the early 1960s (Richardson & Johnson, 1997). The efficacy of these cytotoxic agents is dose-dependent and limited by cardiac tolerability as cardiotoxicity (e.g., cardiomyopathy) is the main adverse effect attributed to anthracyclines (Leonard, Williams, Tulpule, Levine, & Oliveros, 2009). The clinical applications of liposomal-associated anthracyclines either in monotherapy or in combination with other drugs have been well documented by several clinical studies (Schmid et al., 2005; Fridrik et al., 2016; Coltelli et al., 2014; Rigacci et al., 2020). This included reports on maintaining the cytotoxic efficacy whilst minimising cardiotoxicity that permits the use of these chemotherapeutics in broader range of patients with pre-existing cardiopathy.
PEGylated liposomal doxorubicin (PLD) including Doxil® and Caelyx® have also been shown to greatly increase the drug circulation time as a result from the enhanced stability of the drug-loaded liposomal complex (Green & Rose, 2006). This could also be potentially problematic due to slowing the drug release rate, decreasing cytotoxic effects and an increase in liposomal accumulation in skin cells causing hand-foot syndrome (Tse.g., Liu, & Hong, 2002) (Patil, Guhagarkar, & Devarajan, 2008). Aldughaim et al. (2020) formulated a targeted PLD system (Doxil® -P700 complex) by coupling 16-amino acid peptide from tissue inhibitor of metalloproteinase 3 (TIMP3) to the liposomal surface (Aldughaim, Muthana, Alsaffar, & Barker, 2020). This was to specifically target proangiogenic tyrosine kinase receptors. The in vitro results on cancer cells confirmed positive outcomes in relation to the drug targetability which was also hypothesised as an effective approach to reduce Doxil® -P700 complex circulation time in body.
5. Conclusion and future prospects
Nanotechnology has obtained gargantuan volume of researches and developments over the past three decades. A search on PubMed.gov shows around 1, 351 articles published over the applications of nanomedicines in DDS only in 2020. Despite the enormous experimental results from in vitro studies and NPs preparation methodologies in literature, there are still ambiguities around unexplored areas such as in vivo cellular response to nanoparticles and their exact intracellular fate (Zhao et al., 2011). In fact, there is still a lack of reliable in vivo biological data over the toxicological aspects of nanoparticles (Cho et al., 2013; Crucho & Teresa Barros, 2017). This happens to be one of the reasons of not having a massive breakthrough of these products in the pharmaceutical industry as such only a handful of them commercialised to date
(Cho et al., 2013; Crucho & Teresa Barros, 2017). It is important to note however that, reliable characterisation methodology that allows large scale-up production from lab-scale as well as the monitoring of the size and polydispersity index of nanocarriers upon storage is still a challenging task (Danaei et al., 2018). Moreover, multifunctional targeted nanoparticles are a more recent concept that holds promises in cancer chemotherapy (Yu et al., 2010). However, the complexities involved in scale-up productions that meet FDA regulations are all the open challenges (Yu et al., 2010).
Acknowledgements
The authors are grateful to the University of Huddersfield.
Conflict of Interest
The authors note no conflicts of interest.
References
B. A. Kvasnov, (2019). Measurement of the Size and Zeta Potential of Polymer Microspheres Using Dynamic Light Scattering and Electrophoretic Light Scattering Methods: Effect of Viscosity of Dispersion Media. Moscow, DIO: 10.1109/EIConRus.2019.8657244. pp.. pp. 2290–2294.
A. Dalia, (2015). Review article of Nanocarriers Third Generation as Targeted Delivery Systems for Cancer Therapy. s.l.:DOI: 10.13140/RG.2.1.1397.2320.
A. Akbarzadeh, (2013). Liposome: classification, preparation, and applications. The Journal of Nanoscale Research Letters 8(1)
M. S. Aldughaim, M. Muthana, F. Alsaffar, M. D. Barker, (2020). Specific Targeting of PEGylated Liposomal Doxorubicin (Doxil®) to Tumour Cells Using a Novel TIMP3 Peptide. Molecules (Basel, Switzerland) 26(1) p.. : 100.
G. Amoabediny, (2018). Overview of preparation methods of polymeric and lipid-based (niosome, solid lipid, liposome) nanoparticles: A comprehensive review. International Journal of Polymeric Materials, DOI: 10.1080/00914037.2017.1332623 67(6) pp.. : 383–400.
S. Bakand, A. Hayes, (2016). Toxicological Considerations, Toxicity Assessment, and Risk Management of Inhaled Nanoparticles. International Journal of Molecular Sciences 17(6) p.. : 929.
S. Bhattacharjee, (2016). DLS and zeta potential – What they are and what they are not?. The Journal of Controlled Release, DOI:. https://doi.org/10.1016/j.jconrel.2016.06.017 Volume 235 p.. pp. 337–351.
S. Burza, S. L. Croft, M. Boelaert, (2018). Leishmaniasis. The Lancet (British edition) DOI:. https://dx.doi.org/10.1016/S0140-6736(18)31204-2 392(10151) pp.. : 951–970.
J. Buse, A. El-Aneed, (2010). Properties, engineering and applications of lipid-based nanoparticle drug-delivery systems: current research and advances. The Journal of Nanomedicine (Lond) DOI:10.2217/nnm.10.107. Volume 5 pp.. : 1237–60.
E. C. Dreaden, (2012). The golden age: gold nanoparticles for biomedicine. The Journal of Chemical Society Reviews Volume. 41 p.. : 2740–2779.
A. L. P. Cartaxo, (2015). Nanoparticles types and properties – understanding these promising devices in the biomedical area. [Online] Available at:. https://pdfs.semanticscholar.org/9570/b62e051cf8cff1398915cf14eafcff21fbdd.pdf?_ga=2.89239643.1157606762.1595247895-1323696950.1595247895
H. Chen, (2008). Release of hydrophobic molecules from polymer micelles into cell membranes revealed by Forster resonance energy transfer imaging. The Journal of Proceedings of the National Academy of Sciences of the United States of America 105(18) p.. : 6596–6601.
E. J. Cho, (2013). Nanoparticle Characterization: State of the Art, Challenges, and Emerging Technologies. The Journal of Molecular Pharmaceutics Volume. 10 p.. : 2093–2110.
L. Coltelli, (2014). Cardiac Safety Assessment of Adjuvant Nonpegylated Liposomal Doxorubicin (Npld) Plus Cyclophosphamide (C) Followed By Paclitaxel (P) in Elderly Breast Cancer (Ebc) Women. Annals of oncology DOI:10.1093/annonc/mdu327.49. Volume. 25 p. iv101.
C. Crucho, M. Teresa Barros, (2017). Polymeric nanoparticles: A study on the preparation variables andcharacterization methods. The Journal of Materials Science and Engineering C, DOI:. https://doi-org.libaccess.hud.ac.uk/10.1016/j.msec.2017.06.004 Volume 80 pp.. pp. 771–784.
M. Danaei, (2018). Impact of Particle Size and Polydispersity Index on the Clinical Applications of Lipidic Nanocarrier Systems. The Journal of Pharmaceutics DOI:. https://doi.org/10.3390/pharmaceutics10020057 May,. 10(2) p.. : 57.
Y. F. Dufrêne, (2002). Atomic Force Microscopy, a Powerful Tool in Microbiology. The Journal of Bacteriology DOI:. https://dx.doi.org/10.1128%2FJB.184.19.5205–5213.2002 October,. 184(19) p.. : 5205–5213.
K. W. Ford, J. Hornsby, F. Stoutland, K. W. Ford, (2011). 101 Quantum Questions : What You Need to Know about the World You Can't See. Retrieved from. https://ebookcentral.proquest.com Cambridge: Harvard University Press.
M. A. Fridrik, (2016). Cardiotoxicity with rituximab, cyclophosphamide, non-pegylated liposomal doxorubicin, vincristine and prednisolone compared to rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisolone in frontline treatment of patients with diffuse large B-. European journal of cancer (1990), Volume. 58 pp.. pp. 112–121.
A. Gabizon, D. Goren, R. Cohen, Y. Barenholz, (1998). Development of liposomal anthracyclines: from basics to clinical applications. The Journal of Controlled Release DOI:. https://doi-org.libaccess.hud.ac.uk/10.1016/S0168-3659(97)00261-7 53(1-3) p.. : 275–279.
M. Garcia-Fuentes, M. J. Alonso, (2012). Chitosan-based drug nanocarriers: Where do we stand?. The Journal of Controlled Release DOI:. https://doi-org.libaccess.hud.ac.uk/10.1016/j.jconrel.2012.03.017 161(2) p.. : 496–504.
P. Ghasemiyeh, S. Mohammadi-Samani, (2018). Solid lipid nanoparticles and nanostructured lipid carriers as novel drug delivery systems: applications, advantages and disadvantages. The Journal of Research in pharmaceutical sciences 2 July,. 13(4) pp.. : 288–303.
H. Gleiter, (2000). Nanostructured Materials: Basic concepts and microstructure. The Journal of Acta Materialia DOI:. https://doi-org.libaccess.hud.ac.uk/10.1016/S1359-6454(99)00285-2 48(1) pp.. : 1–29.
A. E. Green, P. G. Rose, (2006). Pegylated liposomal doxorubicin in ovarian cancer. International journal of nanomedicine 1(3) pp.. : 229–239.
M. Houshmand, (2020). Nanocarriers as Magic Bullets in the Treatment of Leukemia. The Journal of Nanomaterials DOI:. https://doi.org/10.3390/nano10020276 February,. 10(2) p.. : 276.
C. I. C. Crucho, M. Teresa Barros, (2017). Polymeric nanoparticles: A study on the preparation variables andcharacterization methods. The Journal of Materials Science and Engineering C DOI:. https://doi-org.libaccess.hud.ac.uk/10.1016/j.msec.2017.06.004 Volume. 80 pp.. : 771–784.
F. J. Vidal-Iglesias, (2012). Understanding the Nernst Equation and Other Electrochemical Concepts: An Easy Experimental Approach for Students. The Journal of Chemical Education 89(7) p.. : 936–939.
A. K. Jain, S. Thareja, (2019). In vitro and in vivo characterization of pharmaceutical nanocarriers used for drug delivery. The Journal of Artificial Cells, Nanomedicine, and Biotechnology DOI:. https://doi-org.libaccess.hud.ac.uk/10.1080/21691401.2018.1561457 47(1) pp.. : 524–539.
S. K. Sahoo, V. Labhasetwar, (2003). Nanotech approaches to drug delivery and imaging. The Journal of Drug Discovery Today DOI:. https://doi-org.libaccess.hud.ac.uk/10.1016/S1359-6446(03)02903-9 December,. 8(24) pp.. : 1112–1120.
N. Kamaly, B. Yameen, J. Wu, O. C. Farokhzad, (2016). Degradable Controlled-Release Polymers and Polymeric Nanoparticles: Mechanisms of Controlling Drug Release. The Journal of Chemical Reviews DOI:. https://doi-org.libaccess.hud.ac.uk/10.1021/acs.chemrev.5b00346 116(4) p.. : 2602–2663.
K. Kataoka, A. Harada, Y. Nagasaki, (2001). Block copolymer micelles for drug delivery: design, characterization and biological significance. The Journal of Advanced Drug Delivery Reviews DOI:. https://doi-org.libaccess.hud.ac.uk/10.1016/j.addr.2012.09.013 Volume 47 pp.. : 113–131.
I. Khan, K. Saeed, I. Khan, (2019). Nanoparticles: Properties, applications and toxicities Arabian Journal of Chemistry DOI:. https://doi-org.libaccess.hud.ac.uk/10.1016/j.arabjc.2017.05.011 12(7) pp.. : 908–931.
J. D. Kingsley, (2006). Nanotechnology: A Focus on Nanoparticles as a Drug Delivery System. The Journal of J Neuroimmune Pharmacology DOI:. https://doi-org.libaccess.hud.ac.uk/10.1007/s11481-006-9032-4 Issue. (1) pp.. : 340–350.
S. M. Kullab, P. D. Patel, P. O. Lewis, (2020). Non-occlusive ST-segment elevated myocardial infarction following the administration of liposomal amphotericin B in the treatment of cryptococcal meningitis. Journal of clinical pharmacy and therapeutics 45(5) pp.. : 1168–1171.
R. Kumar MN, (2000). Nano and microparticles as controlled drug delivery devices. The Journal of pharmacy & pharmaceutical sciences 3(2) pp.. : 234–258.
D. Lasic, (1997). Recent developments in medical applications of liposomes: sterically stabilized liposomes in cancer therapy and gene delivery in vivo. The Journal of Controlled Release DOI:. https://doi-org.libaccess.hud.ac.uk/10.1016/S0168-3659(97)00045-X 48(2-3) p.. : 203–222.
S. Laurent, (2008). Magnetic Iron Oxide Nanoparticles: Synthesis, Stabilization, Vectorization, Physicochemical Characterizations, and Biological Applications. The Journal of Chemical Reviews DOI:. https://dx.doi.org/10.1021/cr068445e 108(6) p.. : 2064–2110.
R. Leonard, (2009). Improving the therapeutic index of anthracycline chemotherapy: Focus on liposomal doxorubicin (Myocet™). Breast (Edinburgh) 18(4) pp.. : 218–224.
D. E. Lindquist, L. B. Poveromo, L. M. Vann, R. H. Wrenn, (2020). Liposomal Amphotericin B Infusion–Related Dystonia. The Annals of pharmacotherapy 54(10) pp.. : 1049–1050.
A. Lippacher, R. Müller, K. Mäder, (2000). Investigation on the viscoelastic properties of lipid based colloidal drug carriers. The International Journal of Pharmaceutics 196(2) pp.. : 227–230.
D. Lombardo, M. A. Kiselev, M. Teresa Caccamo, (2019). Smart Nanoparticles for Drug Delivery Application: Development of Versatile Nanocarrier Platforms in Biotechnology and Nanomedicine. The Journal of Nanomaterials DOI:. https://doi.org/10.1155/2019/3702518 February,. Volume. 2019 pp.. : 1–26.
T. M. Allen, (1997). Liposomes Opportunities in Drug Delivery. The Journal of Drugs DOI:. https://dx.doi.org/10.2165/00003495-199700544-00004 October,. 54(S4) p.. : 8–14.
E. M. Martín del Valle, M. A. Galán, R. G. Carbonell, (2009). Drug Delivery Technologies: The Way Forward in the New Decade. The Journal of Industrial and Engineering Chemistry Research DOI:. https://doi-org.libaccess.hud.ac.uk/10.1021/ie800886m 48(5) p.. : 2475–2486.
R. M. Shah, (2014). Physicochemical characterization of solid lipid nanoparticles (SLNs) prepared by a novel microemulsion technique. The Journal of Colloid and Interface Science DOI:. https://dx.doi.org/10.1016/j.jcis.2014.04.057 Volume. 428 p.. : 286–294.
W. Mehnert, K. Mader, (2001). Solid lipid nanoparticles Production, characterization and applications. The Journal of Advanced Drug Delivery Reviews DOI:. https://dx.doi.org/10.1016/s0169-409x(01)00105-3 47(2-3) p.. : 165–196.
K. Miyata, R. J. Christie, K. Kataoka, (2011). Polymeric micelles for nano-scale drug delivery. The Journal of Reactive and Functional Polymers DOI:. https://dx.doi.org/10.1016/j.reactfunctpolym.2010.10.009 71(3) pp.. : 227–234.
S. Moghimi, A. Hunter, J. Murray, (2005). Nanomedicine: current status and future prospects. The Journal of Federation of American Societies for Experimental Biology 19(3) pp.. : 311–330.
C. Mora-Huertas, H. Fessi, A. Elaissari, (2010). Polymer-based nanocapsules for drug delivery. The International Journal of Pharmaceutics DOI:. https://dx.doi.org/10.1016/j.ijpharm.2009.10.018 385(1-2) pp.. : 113–142.
R. Muller, M. Radtke, S. Wissing, (2002). Solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) in cosmetic and dermatological preparations. The Journal of Advanced Drug Delivery Reviews DOI:. https://doi-org.libaccess.hud.ac.uk/10.1016/S0169-409X(02)00118-7 1 November,. Volume. 54 p.. : S131–S155.
N. B. Mutlu-Agardan, C. Sarisozen, V. P. Torchilin, (2020). Cytotoxicity of Novel Redox Sensitive PEG2000-S-S-PTX Micelles against Drug-Resistant Ovarian and Breast Cancer Cells. The Journal of Pharmaceutical Research DOI:. https://doi-org.libaccess.hud.ac.uk/10.1007/s11095-020-2759-4 37(3) p.. : 65.
B. V. Nagavarma Namuri, (2013). Formulation and Evaluation of Polymeric Nanoparticulate Gel for Topical Delivery. International Journal of Polymeric Materials and Polymeric Biomaterials https://dx.doi.org/10.1080/00914037.2013.854213 September,. 63(9) p.. : 439–447.
G. Orive, (2010). Biomaterial-based technologies for brain anti-cancer therapeutics and imaging. The journal of Biochimica et Biophysica Acta 1806(1) pp.. : 96–107.
V. P. Torchilin, (2007). Editorial: Nanocarriers. The Journal of Pharmaceutical Research DOI:. https://dx.doi.org/10.1007/s11095-007-9463-5 December,. 24(12) pp.. : 2333–2334.
V. P. Torchilin, (2012). Multifunctional nanocarriers. The Journal of Advanced Drug Delivery Reviews https://dx.doi.org/10.1016/j.addr.2012.09.031 September,. Volume 64 pp.. : 302–315.
L. P. Balogh, (2010). Why do we have so many definitions for nanoscience and nanotechnology?. The Journal of Nanomedicine: Nanotechnology, Biology, and Medicine 6(3) p.. : 397–398.
S. Parveen, R. Misra, S. K. Sahoo, (2012). Nanoparticles: a boon to drug delivery, therapeutics, diagnostics and imaging. The Journal of Nanomedicine: Nanotechnology, Biology, and Medicine DOI:. 10.1016/j.nano.2011.05.016 8(2) pp.. : 147–166.
R. Patil, S. Guhagarkar, P. Devarajan, (2008). Engineered nanocarriers of doxorubicin: A current update. Crit. Rev. Ther. Drug Carrier Syst. Volume. 25 p.. : 1–61.
C. Pinto Reis, R. J. Neufeld, J. Ribeiro, A. n., F. Veiga, (2006). Nanoencapsulation I. Methods for preparation of drug-loaded polymeric nanoparticles. The Journal of Nanomedicine: Nanotechnology, Biology, and Medicine DOI:. https://dx.doi.org/10.1016/j.nano.2005.12.003 2(1) p.. : 8–21.
J. P. Raoa, K. E. Geckeler, (2011). Polymer nanoparticles: Preparation techniques and size-control parameters. The Journal of Progress in Polymer Science DOI:. https://dx.doi.org/10.1016/j.progpolymsci.2011.01.001 36(7) pp.. : 887–913.
D. Richardson, S. Johnson, (1997). Anthracyclines in haematology: preclinical studies, toxicity and delivery systems. Blood reviews 11(4) pp.. : 201–223.
L. Rigacci, (2020). Nonpeghylated liposomal doxorubicin combination regimen (R-COMP) for the treatment of lymphoma patients with advanced age or cardiac comorbidity. Hematological oncology 38(4) pp.. : 478–486.
M. Roco, (1999). Nanoparticles and nanotechnology research. The Journal of Nanoparticle Research DOI:. https://dx.doi.org/10.1023/A:1010093308079 1(1) pp.. : 1–6.
M. C. Rodríguez Galvis, J. E. Pérez Franco, M. Y. Casas Vargas, M. F. Ordoñez Rubiano, (2020). Effectiveness and Safety of Amphotericin B Deoxycholate, Amphotericin B Colloidal Dispersion, and Liposomal Amphotericin B as Third-Line Treatments for Cutaneous and Mucocutaneous Leishmaniasis: A Retrospective Study. The American journal of tropical medicine and hygiene 102(2) pp.. : 274–279.
P. Schmid, (2005). Primary chemotherapy with gemcitabine as prolonged infusion, non-pegylated liposomal doxorubicin and docetaxel in patients with early breast cancer: final results of a phase II trial. Annals of oncology 16(10) pp.. : 1624–1631.
S. Skoglund, (2017). Difficulties and flaws in performing accurate determinations of zeta potentials of metal nanoparticles in complex solutions—Four case studies. The Journal of Public Library of Science, DOI: 10.1371/journal.pone.0181735 12(7) p.. : e0181735.
C. Suggy S, M. R, A. Imran, (2002). Liposomes (a review)–part two: Drug delivery systems. The Journal of Biopharmaceuticals January,. 15(1) p.. : 40.
R. Tantra, P. Schulze, P. Quincey, (2010). Effect of nanoparticle concentration on zeta-potential measurement results and reproducibility. The Journal of Particuology DOI:. https://dx.doi.org/10.1016/j.partic.2010.01.003 8(3) p.. : 279–285.
The British Standards Institution, B.. Vocabulary: nanoparticles. PAS 71, s.l.: The British Standards Institution(BSI).
V. Torchilin, (2007). Micellar Nanocarriers: Pharmaceutical Perspectives. The Journal of Pharmaceutical Research DOI:. https://dx.doi.org/10.1007/s11095-006-9132-0 24(1) pp.. : 1–16.
Y.-L. Tseng, J.-J. Liu, R.-L. Hong, (2002). Translocation of liposomes into cancer cells by cell-penetrating peptides penetratin and tat: a kinetic and efficacy study. Molecular pharmacology 62(4) pp.. : 864–872.
C. Vauthier, K. Bouchemal, (2009). Methods for the Preparation and Manufacture of Polymeric Nanoparticles. The Journal of Pharmaceutical Research DOI:. https://doi-org.libaccess.hud.ac.uk/10.1007/s11095-008-9800-3 May,. 26(5) pp.. : 1025–1058.
C. Vauthier, P. Couvreur, (2000). Development of nanoparticles made of polysaccharides as novel drug carrier systems. In:. D. Wise, ed.. Handbook of pharmaceutical controlled release technology. New York: Marcel Dekker, p.. pp. 13–429.
Wijnant, G.-J.et al. . (2018). Comparative efficacy, toxicity and biodistribution of the liposomal amphotericin B formulations Fungisome® and AmBisome® in murine cutaneous leishmaniasis. International journal for parasitology – drugs and drug resistance 8(2) pp.. : 223–228.
J. Wingard, (). 2000. Clinical infectious diseases 31(5) pp.. : 1155–1163.
G. Yoon, J. Woo Park, I.-S. Yoon, (2013). Solid lipid nanoparticles (SLNs) and nanostructured lipid carriers (NLCs): recent advances in drug delivery. The Journal of Pharmaceutical Investigation DOI:. https://dx.doi.org/10.1007/s40005-013-0087-y 43(5) p.. : 353–362.
B. Yu, (2010). Receptor-targeted nanocarriers for therapeutic delivery to cancer. The Journal of Molecular Membrane Biology October,. 27(7) p.. : 286–298.
F. Zhao, (2011). Cellular Uptake, Intracellular Traffi cking, and Cytotoxicity of Nanomaterials. The Journal of Small 7(10) p.. : 1322–1337.
M. Zilberman, (2005). Dexamethasone loaded bioresorbable films used in medical support devices: Structure, degradation, crystallinity and drug release. The Journal of Acta Biomaterialia DOI:. https://dx.doi.org/10.1016/j.actbio.2005.06.007 1(6) p.. : 615–624.
A. Zur Mühlen, C. Schwarz, W. Mehnert, (1998). Solid lipid nanoparticles (SLN) for controlled drug delivery – Drug release and release mechanism. European Journal of Pharmaceutics and Biopharmaceutics DOI:. https://dx.doi.org/10.1016/s0939-6411(97)00150-1 45(2) p.. : 149–155.